Getgoal Duo 1

Getgoal Phase Iii Study Programme Download Table
2

Propensity Score Matched Comparative Analyses Of Simultaneously Administered Fixed Ratio Insulin Glargine 100 U And Lixisenatide Iglarlixi Vs Sequential Administration Of Insulin Glargine And Lixisenatide In Uncontrolled Type 2 Diabetes Rosenstock
Http Www Siditalia It Pdf Relazioni Liguria Congresso 17 Sesti Pdf

Pdf Prandial Options To Advance Basal Insulin Glargine Therapy Testing Lixisenatide Plus Basal Insulin Versus Insulin Glulisine Either As Basal Plus Or Basal Bolus In Type 2 Diabetes The Getgoal Duo 2 Trial Semantic

Pdf Lixisenatide As Add On Therapy To Basal Insulin
In the GetGoal-Duo-1 trial, both groups gained weight, but patients in the lixisenatide arm gained less than those in the control arm (0.3 kg versus 1.2 kg, respectively;.

Getgoal duo 1. Efficacy and Safety of Lixisenatide Versus Placebo on Top of Basal Insulin and/or Oral Antidiabetic Treatment in Older Type 2 Diabetic Patients (GetGoal-O) The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. The originality of GetGoal DUO 2 is the comparison between add-on of lixisenatide and active treatment (prandial insulin). Efficacy and safety of once-daily lixisenatide added on to titrated glargine plus oral agents in type 2 diabetes:.
Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:. Riddle MC, Forst T, Aronson R, et al. In GetGoal Duo 1, lixisenatide in combination with insulin glargine achieved the primary study endpoint of significantly reducing HbA1c with a significant improvement in 2-hour post-prandial.
The GetGoal Duo-2 Trial. The GetGoal program started in May 08 and has enrolled more than 4,500 patients. A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1).
Again, as in the 4B, body weight and hypoglycemia incidence. Propensity Score Matched Analysis. The GetGoal Duo 1 study evaluated once-daily lixisenatide added on to titrated glargine plus oral agents in type 2 diabetes.
In the GetGoal Duo 1, randomized, double-blind, multicenter study, 8 insulin-naïve patients were treated with insulin glargine (Lantus), which was titrated to reach a target fasting plasma glucose (FPG) of 80-100 mg/dL for a 12-week run-in phase period. Riddle MC, Forst T, Aronson R, Sauque-Reyna L, Souhami E, Silvestre L, Ping L, Rosenstock J. The GetGoal-Duo 1 study ePoster # 807 Session:.
OBJECTIVE To provide evidence-based options on how to intensify basal insulin, we explored head-to-head prandial interventions in overweight patients with type 2 diabetes inadequately controlled on basal insulin glargine with or without 1–3 oral antidiabetic agents (OADs). Patients in GetGoal-Duo 1 were from 25 countries and were inadequately controlled (HbA1c 53–86 mmol/mol 7.0–10.0%) on existing OAD therapy. Change in 2-h PPG and glucose.
Plain language summary available for this article. These patients were on existing basal insulin therapy with or without a SU. PS 063 GLP-1 based therapies Berlin 12 Poster Hall 3.
The abstract is titled:. To date, GetGoal-X, GetGoal-L, GetGoal-L Asia, GetGoal-Mono, GetGoal-S, GetGoal-F1 and GetGoal Duo 1 (also known as EFC*) have reported positive top-line results supporting potential efficacy and safety for lixisenatide. Like in 4B, in GetGoal DUO 2 the A1C decreased to similar values with lixisenatide or glulisine 1/d (~7.2%), or glulisine 3/d (~7.0%).
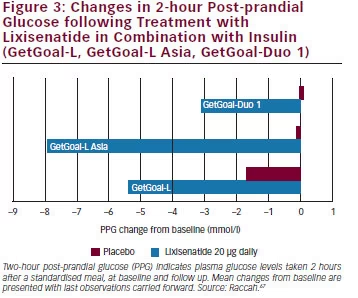
For Type 2 diabetes patients treated with basal. GetGoal Duo 1 and GetGoal-L both achieved the primary efficacy endpoint of HbA1c improvement with an associated significant reduction in PPG. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:.
A 24- week, Randomized, Placebo-Controlled Study (GetGoal Duo 1).” Diabetes Care 36.9 (13):. GetGoal-M, GetGoal-S) or in combination with basal insulin (GetGoal-L, GetGoal-Duo-1 and GetGoal-L-Asia). Rosenstock J, Forst T, Aronson R, et al.
Sanofi announced positive results from its Phase 3, GetGoal Duo 1 study of Lyxumia (lixisenatide) in combination with Lantus (insulin glargine;. Epub 16 May 23. Six randomized, placebo-controlled studies of lixisenatide µg once daily were included in this analysis:.
Sanofi) and OADs. A two-step dosage increase was used with both placebo and lixisenatide (10 μg for 1 week, 15 μg for 1 week, and then -μg maintenance dosage if tolerated), with injections self-administered by participants ≤1 h before breakfast. A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1).
Once-daily lixisenatide added on to consistently titrated insulin glargine plus oral agents in type 2 diabetes:. To conduct two exploratory analyses to compare indirectly the efficacy and safety of simultaneous administration of insulin glargine 100 U (iGlar) and the glucagon-like peptide-1 receptor agonist (GLP-1RA) lixisenatide (Lixi) as a single-pen, titratable, fixed-ratio combination (iGlarLixi LixiLan trials) vs sequential administration of iGlar + Lixi (GetGoal Duo trials) in people with. Efficacy and Safety of Lixisenatide Versus Insulin Glulisine on Top of Insulin Glargine With or Without Metformin in Type 2 Diabetic Patients (GetGoal-Duo-2) The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
The GetGoal Duo-2 Evidence-Based Trial (NCT). The GetGoal program was initiated in May 08. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:.
Lixisenatide improved HbA1c, weight with standard GLP-1 safety profile PHILADELPHIA — Once-daily lixisenatide, an investigational GLP-1 agonist, was associated with significant HbA1c. "During a 12-week run-in phase, 8 insulin-naive patients were. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:.
October 12 12:00 - 13:00. 21 – 26 The GetGoal-X trial, which compared lixisenatide with twice-daily exenatide, resulted in weight loss of 2.96 kg and 3.98 kg, respectively. GetGoal Duo 1 was a randomized, double-blind, multicenter study assessing the efficacy and safety of lixisenatide compared to placebo in combination with Lantus (insulin glargine;.
"The results from the GetGoal Duo-2 study reconfirm the therapeutic benefits of Lyxumia(R) as a novel prandial GLP-1 agonist. A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). During the 12-week run-in phase, 8 insulin-naive patients were treated with insulin glargine, which was titrated to reach a target fasting.
Patients were randomized to receive lixisenatide or placebo 1:1 in GetGoal-Duo1 and GetGoal-L-Asia, and 2:1 in GetGoal-L. Riddle MC, Forst T, Aronson R et al. NCT (GetGoal Duo-2 trial).
Riddle, Mattew C.et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:. Testing Lixisenatide Plus Basal Insulin Versus Insulin Glulisine Either as Basal-Plus or Basal-Bolus in Type 2 Diabetes:.
Efficacy and Safety of iGlarLixi, Fixed-Ratio Combination of Insulin Glargine and Lixisenatide, Compared with Basal-Bolus Regimen in Patients with Type 2 Diabetes:. Prandial Options to Advance Basal Insulin Glargine Therapy:. Sanofi) for the treatment of patients with type 2.
The trials were conducted between July 08 and August 11 across 25 countries (the number of countries and enrolment/completion dates varied by trial). Patients in GetGoal-Duo 1 were from 25 countries and were inadequately controlled (HbA1c 53–86 mmol/mol 7.0–10.0%) on existing OAD therapy. Riddle MC, Forst T, Aronson R, et al.
Duo is the highest quality 1 video calling app. If present, SU therapy was discontinued, and patients were initiated on basal insulin therapy with or without MET or a TZD during the run-in phase. So far, GetGoal-X, GetGoal-L, GetGoal-L Asia, GetGoal-Mono, GetGoal-S, GetGoal-F1 and GetGoal Duo 1 have reported positive top-line results.
Results showed that lixisenatide caused mild and transient nausea and vomiting, the most common adverse events, and a limited additional or comparable risk of hypoglycemia. Therefore, propensity score matching was used to indirectly compare simultaneous administration of iGlarLixi in the LixiLan-O trial (n=469) with sequential therapy, starting with initial insulin glargine 100 U/mL therapy for 12 weeks, followed by addition of lixisenatide in the GetGoal Duo-1 trial in patients with Type 2 diabetes mellitus who. Presented at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia PA, 8-12 June 12 (Abstract 62-OR).
-1 0 1 GetGoal-L1 GetGoal-L Asia2 GetGoal-Duo 13 e ) ~16.0 (2 mg/dL) 17.8 (3.4 mg/dL) ~13.0 (234 mg/dL) Baseline PPG Lixisenatide Added to Background Insulin:. Adjustment of dosage of insulin glargine. Lixisenatide activates the GLP-1 receptor and thereby exercises the range of physiological effects generated by GLP-1, which consist of increased insulin secretion, inhibition of glucagon secretion, and decreased gastrointestinal motility alongside the promotion of.
Lantus(R) is the No.1 leading basal insulin product in the world, and the results from GetGoal Duo 1 show that adding lixisenatide to treatment with Lantus(R) can offer significant benefits to patients." "Lixisenatide is a promising new GLP-1 agonist with a mode of action which complements that of basal insulin. MC Riddle, T Forst, R Aronson, et al.Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:. The GetGoal Duo-1 trial was the first study to assess the efficacy of lixisenatide in combination with optimally titrated basal insulin in patientswith type2 diabe-tesuncontrolledonOADswhowerenewly initiatinginsulinglargine.Signi ficantreduc-tions in HbA 1c to 7% (53 mmol/mol) and marked PPG reductions were achieved.
Lixisenatide as monotherapy (GetGoal-Mono), as add-on to oral antidiabetic drugs (OADs;. After a 12-week run-in phase in which insulin glargine was initiated, patients with A1C ≥7% were randomized to μg lixisenatide ( n = 223) or placebo ( n = 223) for 24 weeks while continuing on insulin glargine. GetGoal DUO 2 follows the studies GetGoal-L and GetGoal DUO 1 , which have both explored the efficacy and safety of once-a-day add-on of lixisenatide to basal insulin at fixed dose , or to insulin glargine with continuing titration as compared to placebo.
Advancing Basal Insulin Glargine with Prandial Lixisenatide QD vs Insulin Glulisine QD or TID in T2DM:. Further results are expected in 12. “Adding Once – Daily Lixisenatide for Type 2 Diabetes Inadequately Controlled With Newly Initiated and Continuously Titrated Basal Insulin Glargine:.
Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:. A 24-week, randomized, placebo-controlled study. RIDDLE THOMAS FORST RONNIE ARONSON FRCPC FACE LEOBARDO SAUQUE-REYNA ELISABETH SOUHAMI LOUISE SILVESTRE LIN PING JULIO ROSENSTOCK A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1) OBJECTIVEdWhen oral therapy for type 2 diabetes is ineffective, adding basal insulin improves glycemic control.
Patients in GetGoal-L Asia were from Japan, Republic of Korea, Taiwan, and the Philippines. In conclusion, 1) GLP-1-(7-36) amide or -(7-37) inhibits gastric emptying also in normal subjects, 2) physiological doses (0.4 pmol.kg-1.min-1) still have a significant effect, 3) despite the. The glucagon-like peptide (GLP)-1 receptor agonist lixisenatide (Lyxumia®) was approved for marketing by the European Medicines Agency in February 13 and has been evaluated in a clinical study program called GetGoal.
GetGoal Duo 1 is a randomized, double-blind, multicenter study, assessing the efficacy and safety of lixisenatide, compared to placebo, in combination with insulin glargine and OADs (mostly metformin). Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:. A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1).
A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). The GetGoal-Duo 1 study assessed the complementary action of lixisenatide and insulin glargine in patients with type 2 diabetes failing on oral antidiabetes medication. Secondary Endpoints Change in 2-hr PPG Change in Body Weight.
A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1).
It’s free, simple and works on Android phones, iPhones, tablets, computers, and smart displays, like the Google Nest Hub Max. A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine:.
GETGOAL GLP-1 agonist AVE0010 in patients with type 2 diabetes mellitus for glycaemic control and safety evaluation (lixisenatide, µg once daily), GLP-1 RA glucagon-like peptide-1 receptor agonist,. In GetGoal DUO 2, the head-to-head comparison was between lixisenatide 1/d vs glulisine either 1/d (at the main meal, basal-plus) or 3/d (basal-bolus). GETGOAL-L-Asia GETGOAL-Duo-1 Cardiovascular outcome trials for GLP-1 RAs.

Intensification Of Basal Insulin Therapy With Lixisenatide In Patients With Type 2 Diabetes In A Real World Setting The Basal Lixi Study Sciencedirect
2

Pdf Consensus Recommendations On Glp 1 Ra Use In The Management Of Type 2 Diabetes Mellitus South Asian Task Force Semantic Scholar

Session Two Changing The Type 2 Diabetes Mellitus Management Paradigm With Fixed Ratio Combinations European Medical Journal

Table 4 From Efficacy And Safety Of Lixisenatide In Elderly 65 Years Old And Very Elderly 75 Years Old Patients With Type 2 Diabetes An Analysis From The Getgoal Phase Iii Programme Semantic Scholar

Predisposing Factors For Any And Major Hypoglycemia With Saxagliptin Versus Placebo And Overall Analysis From The Savor Timi 53 Trial Diabetes Care

Is There A Justification For Classifying Glp 1 Receptor Agonists As Basal And Prandial Topic Of Research Paper In Clinical Medicine Download Scholarly Article Pdf And Read For Free On Cyberleninka Open

The Clinical Development Program Of Lixisenatide A Once Daily Glucagon Like Peptide 1 Receptor Agonist Abstract Europe Pmc

Challenges And Opportunities In The Treatment Of Type 2 Diabetes Nancy A Thornberry Pdf Free Download

Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine Diabetes Care

Full Text Lixisenatide As Add On Therapy To Basal Insulin Dddt

Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine Diabetes Care

Study Design Of Getgoal Duo 1 16 Download Scientific Diagram
Http Medicoinvestor Com Wp Content Uploads 12 12 Lyxumia Pdf

Pdf Lixisenatide As Add On Therapy To Basal Insulin Semantic Scholar

The Rationale For Combining Glp 1 Receptor Agonists With Basal Insulin Cohen 13 Medical Journal Of Australia Wiley Online Library

Initiating Injectable Treatment Of Type 2 Diabetes A Focus On Glucagon Like Peptide 1 Receptor Agonists Consultant360

The Clinical Development Program Of Lixisenatide A Once Daily Glucagon Like Peptide 1 Receptor Agonist Abstract Europe Pmc

Combination Therapy Of Glucagon Like Peptide 1 Receptor Agonists And Insulin For Patients Who Developed Diabetes After Partial Pancreatectomy Kitazawa 16 Journal Of Diabetes Investigation Wiley Online Library
Lixisenatide A New Glucagon Like Peptide 1 Receptor Agonist In The Treatment Of Type 2 Diabetes Touchendocrinology

Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine Diabetes Care
Www Sanofi Com Media Project One Sanofi Web Websites Global Sanofi Com Home En Investors Docs I P Ir Call Ada15 Final Pdf La En Hash 53cad627a4edf9ebcb3ed

Lixisenatide Plus Basal Insulin In Patients With Type 2 Diabetes Mellitus A Meta Analysis Sciencedirect
Gale Academic Onefile Document Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine A 24 Week Randomized Placebo Controlled Study Getgoal Duo 1

Efficacy And Safety Of Short And Long Acting Glucagon Like Peptide 1 Receptor Agonists On A Background Of Basal Insulin In Type 2 Diabetes A Meta Analysis Diabetes Care

Efficacy And Safety Of Lixilan A Titratable Fixed Ratio Combination Of Lixisenatide And Insulin Glargine Versus Insulin Glargine In Type 2 Diabetes Inadequately Controlled On Metformin Monotherapy The Lixilan Proof Of Concept Randomized Trial
Http Www Siditalia It Pdf Relazioni Liguria Congresso 17 Sesti Pdf
Http Clinical Diabetesjournals Org Content Early 18 02 05 Cd17 0048 Full Text Pdf
Simultaneous Vs Sequential Combination Of Insulin Glargine And Lixisenatide In Type 2 Diabetes Uncontrolled On Metformin Virtual Meeting Easd

Beyond Basal Insulin How Glucagon Like Peptide 1 Receptor Agonists Fit In The Spectrum Of Therapeutic Options Consultant360

Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine A 24 Week Ppt Download
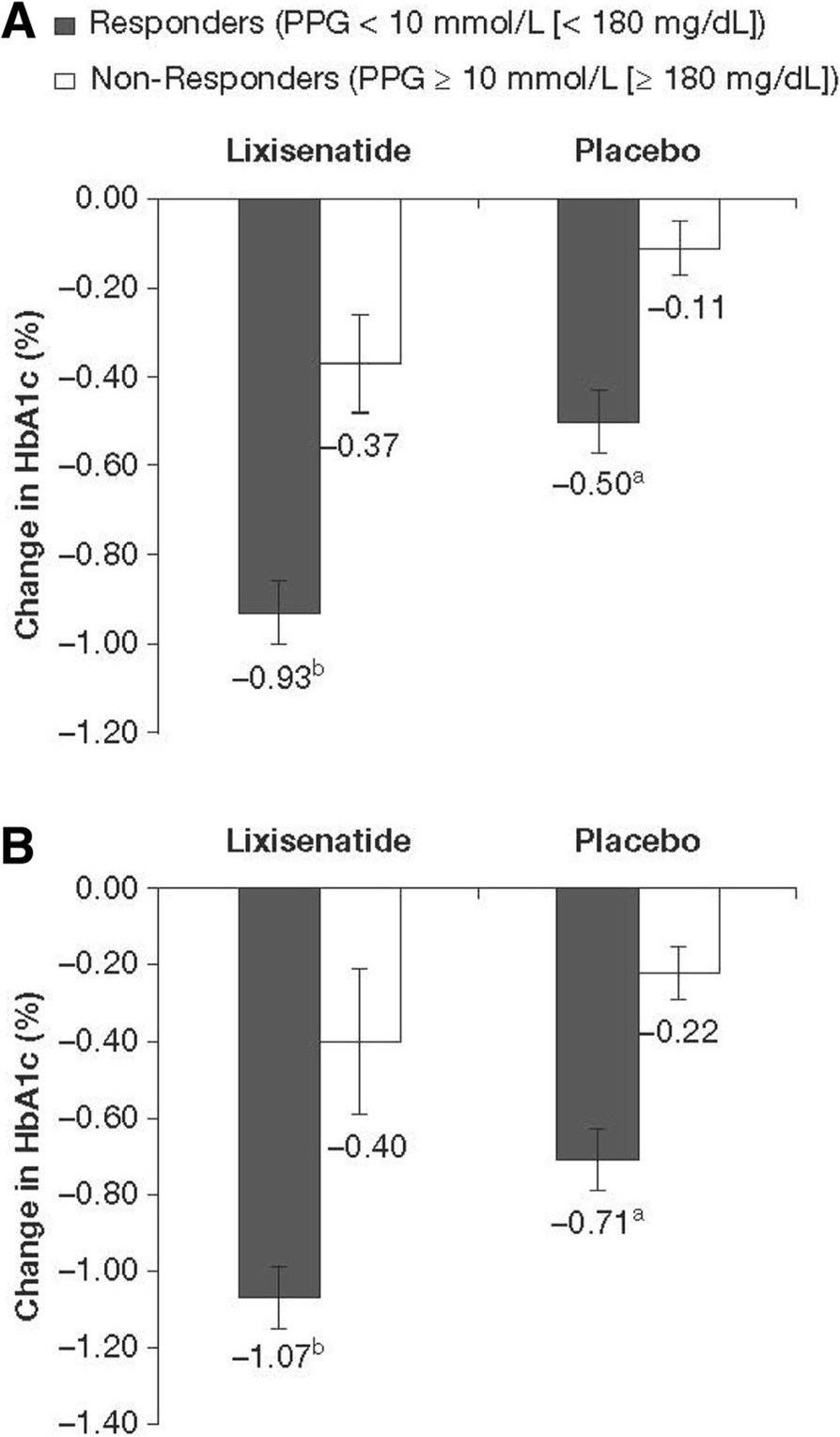
Achieving Postprandial Glucose Control With Lixisenatide Improves Glycemic Control In Patients With Type 2 Diabetes On Basal Insulin A Post Hoc Analysis Of Pooled Data Springerlink
Lixisenatide In Type 2 Diabetes Latest Evidence And Clinical Usefulness Abstract Europe Pmc

Table 4 From Efficacy And Safety Of Lixisenatide In Elderly 65 Years Old And Very Elderly 75 Years Old Patients With Type 2 Diabetes An Analysis From The Getgoal Phase Iii Programme Semantic Scholar
2

Lyxumia Lixisenatide In Combination With Basal Insulin Plus Oral Anti Diabetics Significantly Reduced Hba1c And Post Prandial Glucose

Composite Of A Glycated Haemoglobin Hba1c B Fasting Plasma Glucose Download Scientific Diagram
2
Lixisenatide A New Glucagon Like Peptide 1 Receptor Agonist In The Treatment Of Type 2 Diabetes Touchendocrinology

Reviewer Worksheets Pharmacoeconomic Review Report Lixisenatide Adlyxine Ncbi Bookshelf
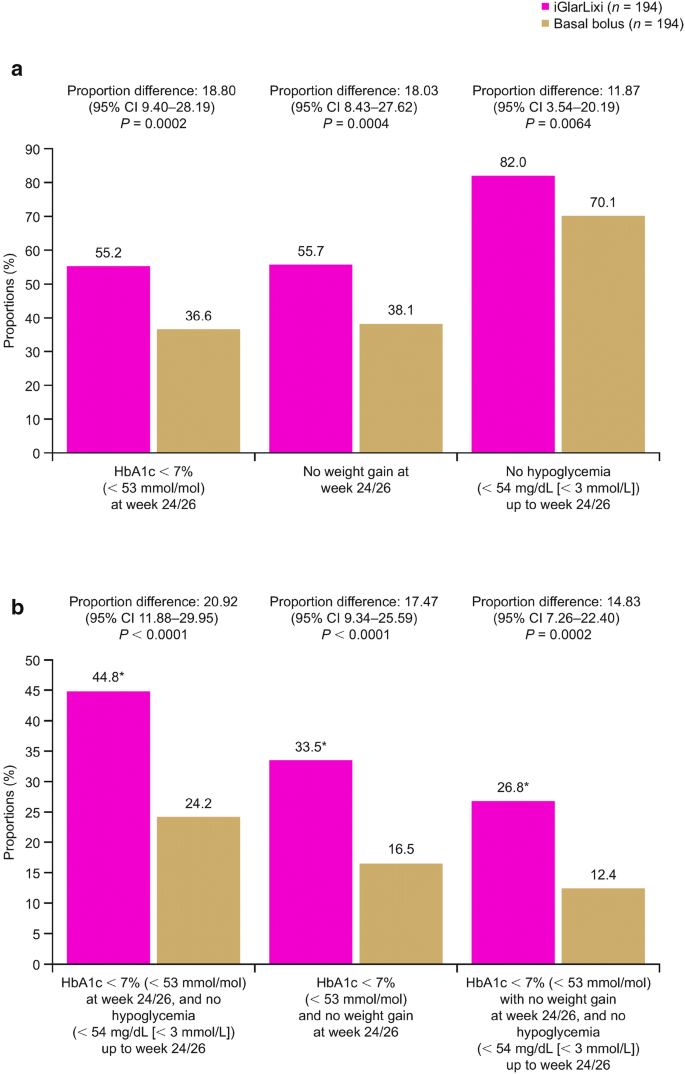
Efficacy And Safety Of Iglarlixi Fixed Ratio Combination Of Insulin Glargine And Lixisenatide Compared With Basal Bolus Regimen In Patients With Type 2 Diabetes Propensity Score Matched Analysis Springerlink

Study Design Of Getgoal Duo 1 16 Download Scientific Diagram
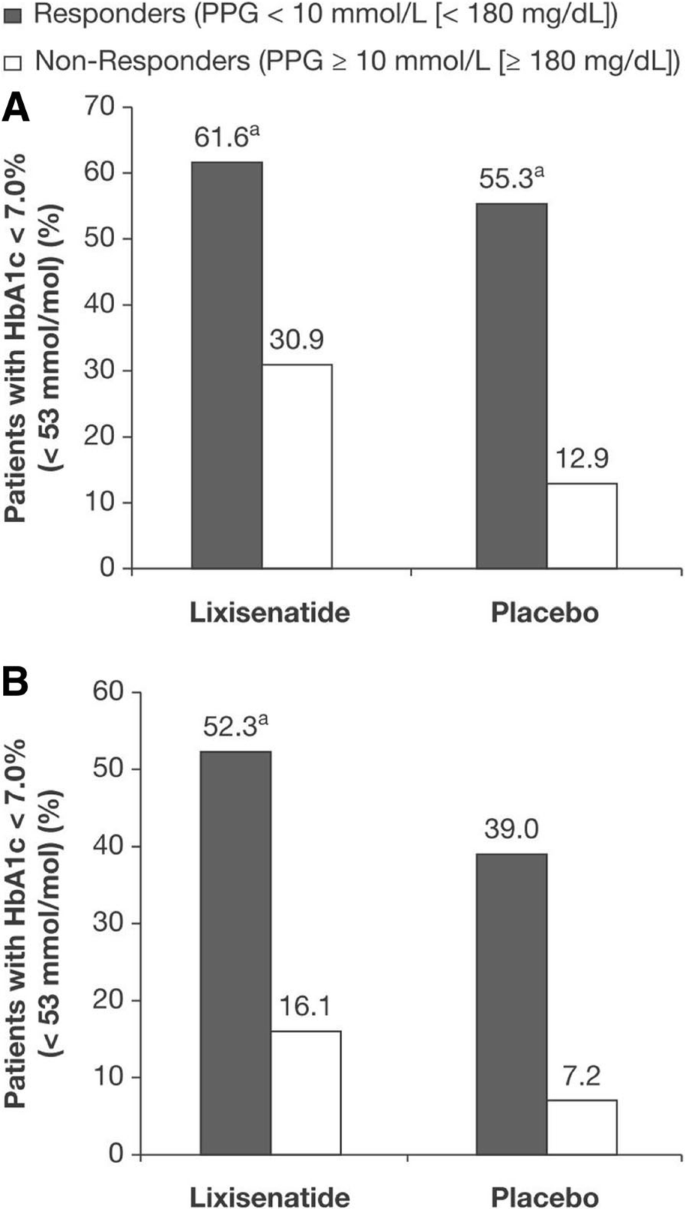
Achieving Postprandial Glucose Control With Lixisenatide Improves Glycemic Control In Patients With Type 2 Diabetes On Basal Insulin A Post Hoc Analysis Of Pooled Data Clinical Diabetes And Endocrinology Full Text
Gale Academic Onefile Document Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine A 24 Week Randomized Placebo Controlled Study Getgoal Duo 1

Prandial Options To Advance Basal Insulin Glargine Therapy Testing Lixisenatide Plus Basal Insulin Versus Insulin Glulisine Either As Basal Plus Or Basal Bolus In Type 2 Diabetes The Getgoal Duo 2 Trial Diabetes Care
2
Results Clinical Review Report Lixisenatide Adlyxine Ncbi Bookshelf

Pdf Prandial Options To Advance Basal Insulin Glargine Therapy Testing Lixisenatide Plus Basal Insulin Versus Insulin Glulisine Either As Basal Plus Or Basal Bolus In Type 2 Diabetes The Getgoal Duo 2 Trial Semantic

Efficacy And Safety Of Lixisenatide In Elderly 65 Years And Very Elderly 75 Years Patients With Type 2 Diabetes An Analysis From The Getgoal Phase 3 Programme Virtual Meeting Easd

Lixisenatide Plus Basal Insulin In Patients With Type 2 Diabetes Mellitus A Meta Analysis Sciencedirect

The Clinical Development Program Of Lixisenatide A Once Daily Glucagon Like Peptide 1 Receptor Agonist Topic Of Research Paper In Clinical Medicine Download Scholarly Article Pdf And Read For Free On Cyberleninka Open Science

Table 4 From Efficacy And Safety Of Lixisenatide In Elderly 65 Years Old And Very Elderly 75 Years Old Patients With Type 2 Diabetes An Analysis From The Getgoal Phase Iii Programme Semantic Scholar
Pdf Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine A 24 Week Randomized Placebo Controlled Study Getgoal Duo 1
Add On Options To Basal Insulin Targeting Ppg In Type 2 Diabetes Patients Transcript

A Systematic Review And Meta Analysis Of The Efficacy Of Lixisenatide In The Treatment Of Patients With Type 2 Diabetes Schmidt 14 Diabetes Obesity And Metabolism Wiley Online Library

The Role Of Glp 1 Receptor Agonists As Weight Loss Agents In Patients With And Without Type 2 Diabetes Dar 15 Practical Diabetes Wiley Online Library

Intensification Of Basal Insulin Therapy With Lixisenatide In Patients With Type 2 Diabetes In A Real World Setting The Basal Lixi Study Sciencedirect
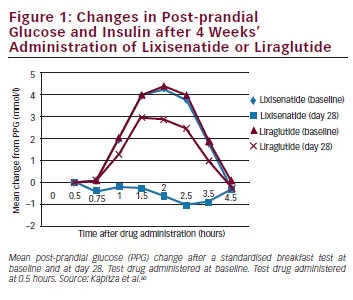
Lixisenatide A New Glucagon Like Peptide 1 Receptor Agonist In The Treatment Of Type 2 Diabetes Touchendocrinology

Pdf Injectable Coformulations In Diabetology Semantic Scholar

Benefits Of Lixilan A Titratable Fixed Ratio Combination Of Insulin Glargine Plus Lixisenatide Versus Insulin Glargine And Lixisenatide Monocomponents In Type 2 Diabetes Inadequately Controlled On Oral Agents The Lixilan O Randomized Trial
Http Journals Sagepub Com Doi Pdf 10 1177
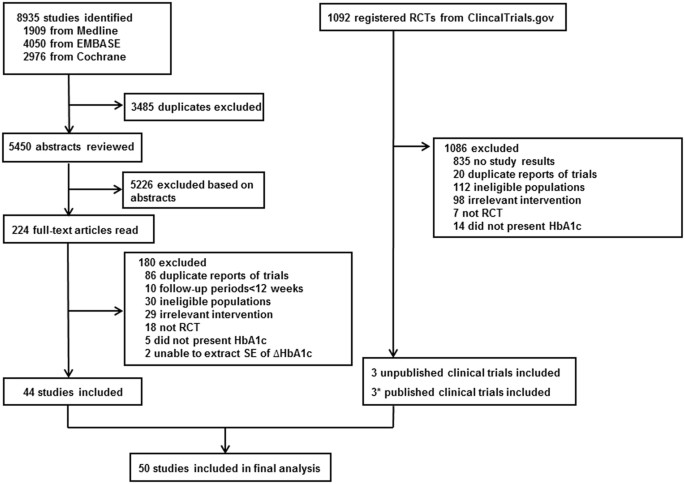
Comparison Of Non Insulin Antidiabetic Agents As An Add On Drug To Insulin Therapy In Type 2 Diabetes A Network Meta Analysis Scientific Reports

Full Text Lixisenatide As Add On Therapy To Basal Insulin Dddt
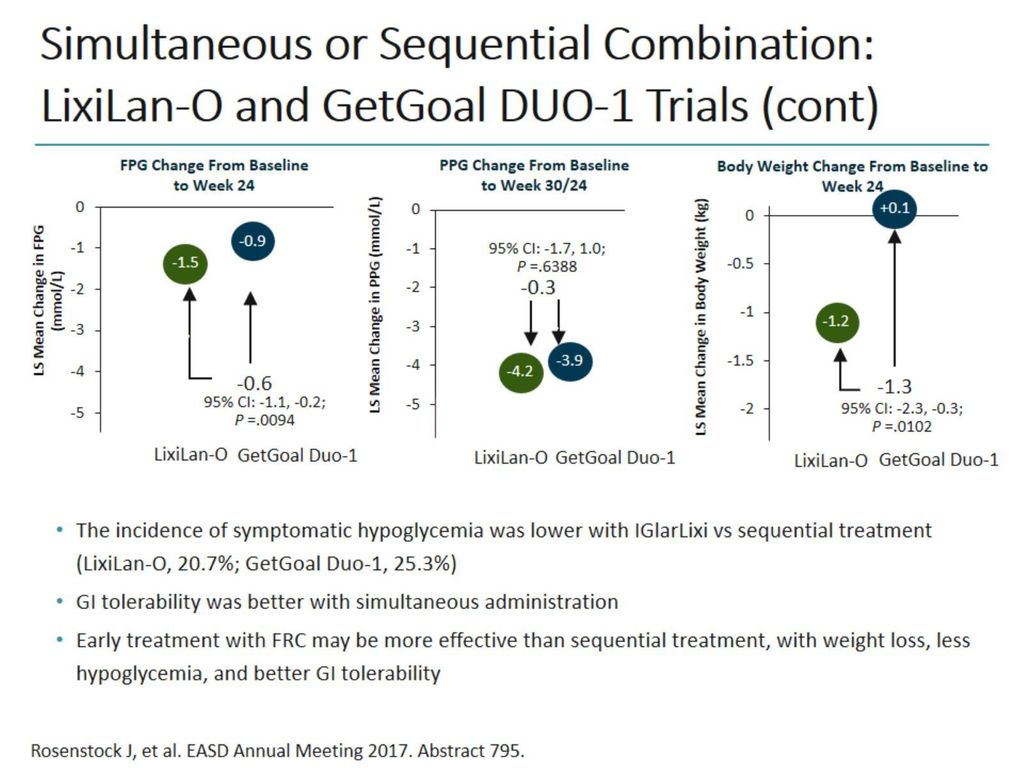
Insulin Glp 1 Agonist Combinations Ppt Download

Pdf Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine A 24 Week Randomized Placebo Controlled Study Getgoal Duo 1

New Forms Of Insulin And Insulin Therapies For The Treatment Of Type 2 Diabetes The Lancet Diabetes Endocrinology

Full Text Lixisenatide As Add On Therapy To Basal Insulin Dddt

Once Daily Lixisenatide Added On To Consistently Titrated Insulin Glargine Plus Oral Agents In Type 2 Diabetes The Getgoal Duo 1 Study Virtual Meeting Easd

Supplemental Materials For Glucagon Like Peptide 1 Receptor Agonist And Basal Insulin Combination Treatment For The Management Of Type 2 Diabetes A Systematic Review And Meta Analysis The Lancet

Pdf The Efficacy And Safety Of Lixisenatide In A Predominantly Asian Population With Type 2 Diabetes Insufficiently Controlled With Basal Insulin The Getgoal L C Randomized Trial

Session Two Changing The Type 2 Diabetes Mellitus Management Paradigm With Fixed Ratio Combinations European Medical Journal
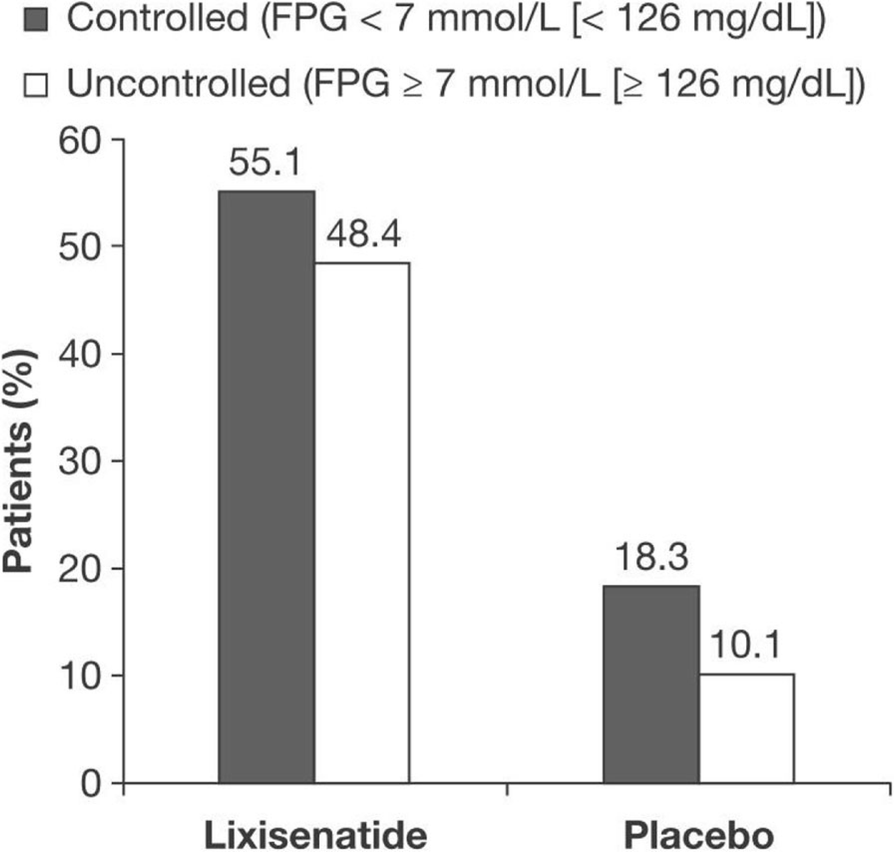
Achieving Postprandial Glucose Control With Lixisenatide Improves Glycemic Control In Patients With Type 2 Diabetes On Basal Insulin A Post Hoc Analysis Of Pooled Data Springerlink

Prandial Options To Advance Basal Insulin Glargine Therapy Testing Lixisenatide Plus Basal Insulin Versus Insulin Glulisine Either As Basal Plus Or Basal Bolus In Type 2 Diabetes The Getgoal Duo 2 Trial Diabetes Care

Study Design Of Getgoal Duo 1 16 Download Scientific Diagram
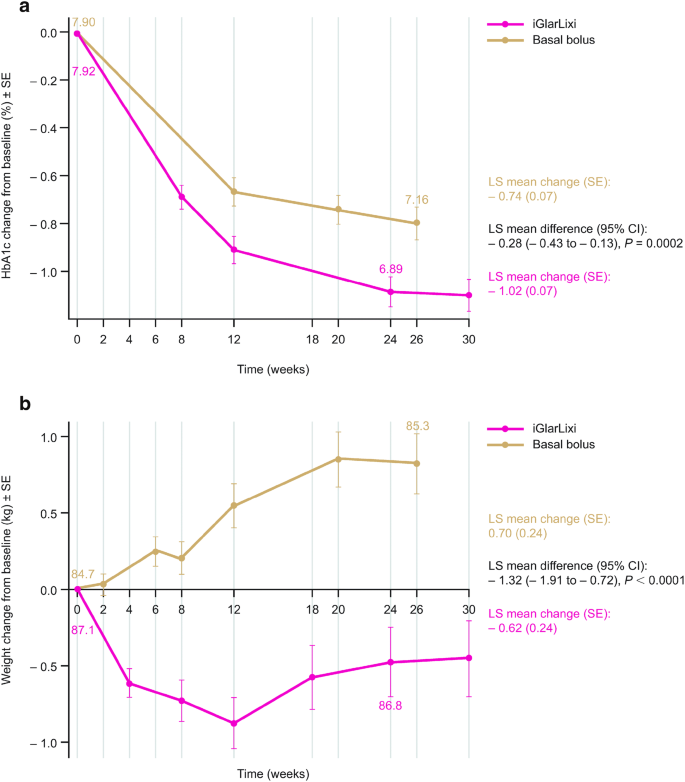
Efficacy And Safety Of Iglarlixi Fixed Ratio Combination Of Insulin Glargine And Lixisenatide Compared With Basal Bolus Regimen In Patients With Type 2 Diabetes Propensity Score Matched Analysis Springerlink

Once Daily Lixisenatide Added On To Consistently Titrated Insulin Glargine Plus Oral Agents In Type 2 Diabetes The Getgoal Duo 1 Study Virtual Meeting Easd
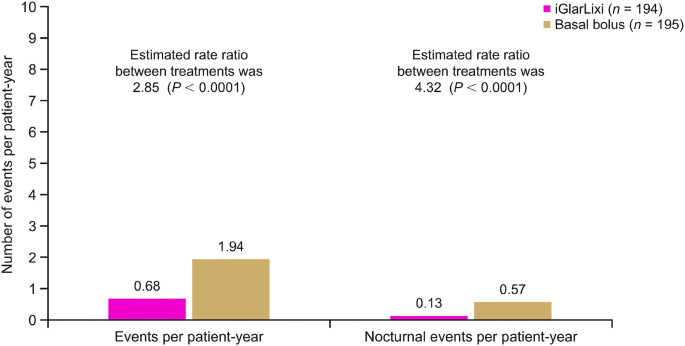
Efficacy And Safety Of Iglarlixi Fixed Ratio Combination Of Insulin Glargine And Lixisenatide Compared With Basal Bolus Regimen In Patients With Type 2 Diabetes Propensity Score Matched Analysis Springerlink
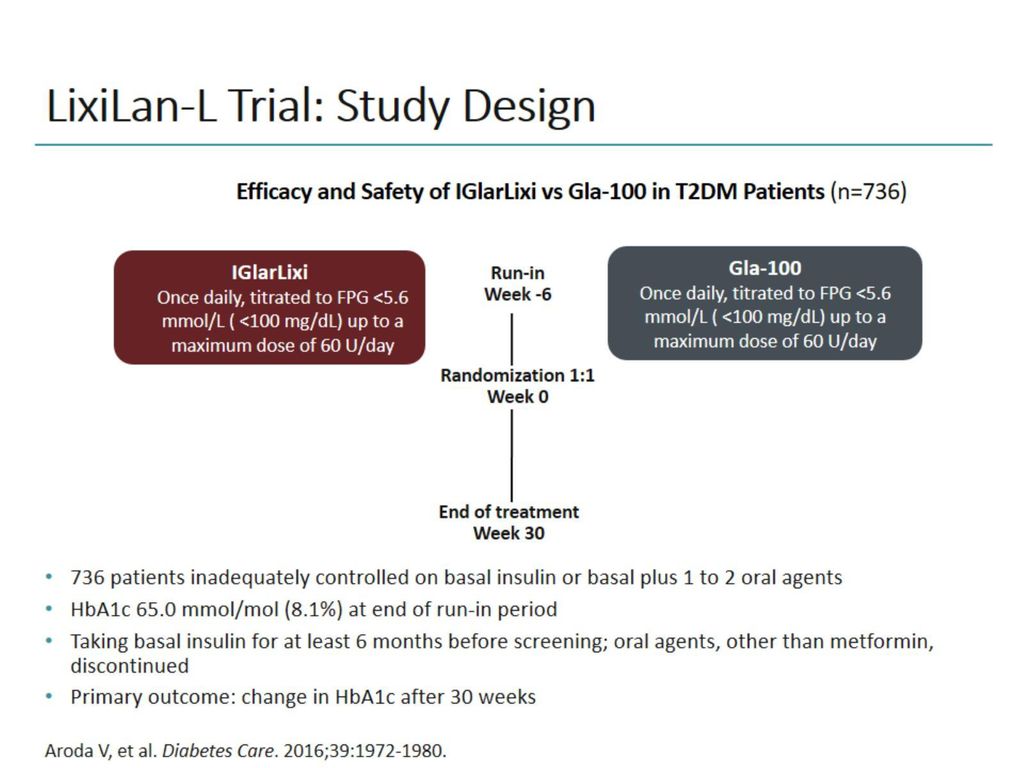
Insulin Glp 1 Agonist Combinations Ppt Download

Initiating Injectable Treatment Of Type 2 Diabetes A Focus On Glucagon Like Peptide 1 Receptor Agonists Consultant360

Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine Diabetes Care
Full Article Lixisenatide As Add On To Insulin Glargine For The Treatment Of Type 2 Diabetes Mellitus

Switching From Insulin Bolus Treatment To Glp 1 Ras Added To Continued Basal Insulin In People With Type 2 Diabetes On Basal Bolus Insulin Diabetes Care
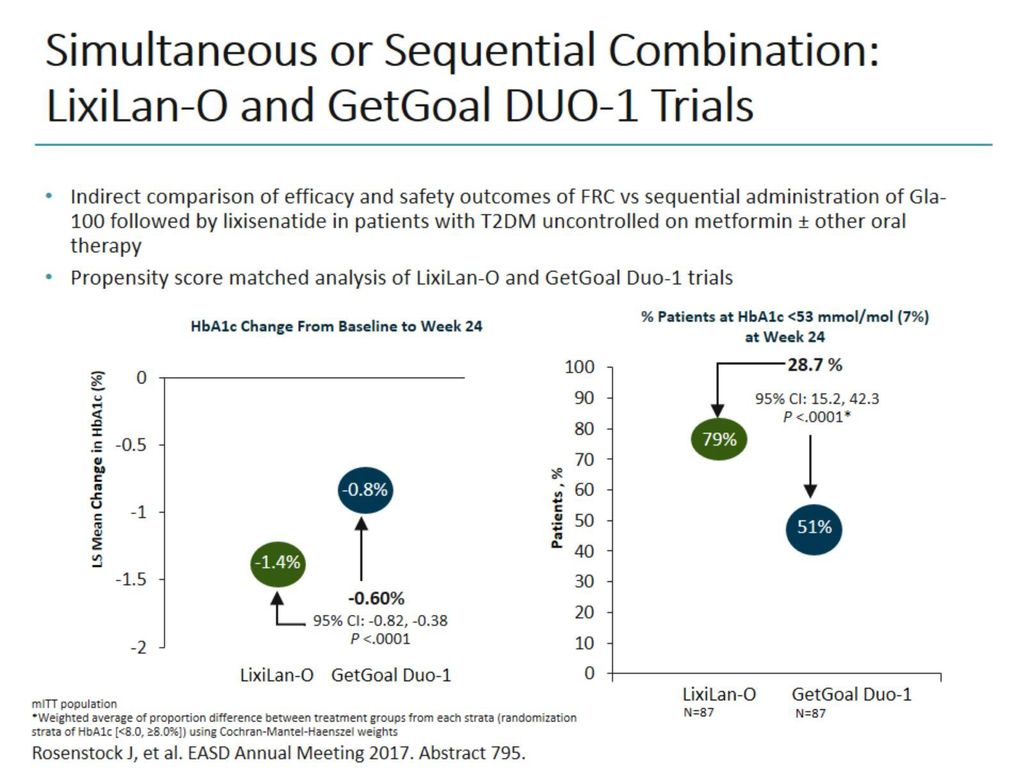
Insulin Glp 1 Agonist Combinations Ppt Download

Pdf Adding Once Daily Lixisenatide For Type 2 Diabetes Inadequately Controlled With Newly Initiated And Continuously Titrated Basal Insulin Glargine A 24 Week Randomized Placebo Controlled Study Getgoal Duo 1

Propensity Score Matched Comparative Analyses Of Simultaneously Administered Fixed Ratio Insulin Glargine 100 U And Lixisenatide Iglarlixi Vs Sequential Administration Of Insulin Glargine And Lixisenatide In Uncontrolled Type 2 Diabetes Abstract

Table 3 From Efficacy And Safety Of Lixisenatide In Elderly 65 Years Old And Very Elderly 75 Years Old Patients With Type 2 Diabetes An Analysis From The Getgoal Phase Iii Programme Semantic Scholar

Composite Of A Glycated Haemoglobin Hba1c B Fasting Plasma Glucose Download Scientific Diagram
Citeseerx Ist Psu Edu Viewdoc Download Doi 10 1 1 1054 3095 Rep Rep1 Type Pdf

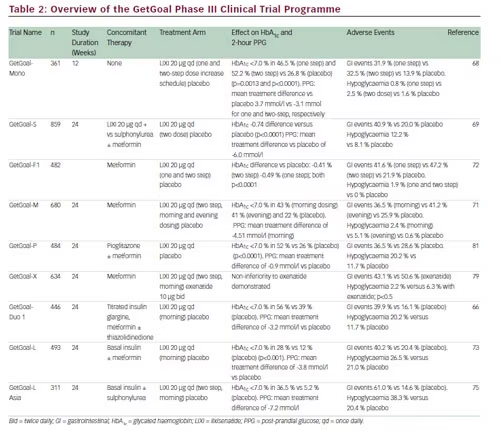
Summary Of The Getgoal Phase Iii Clinical Trial Program With Lixisenatide Download Table
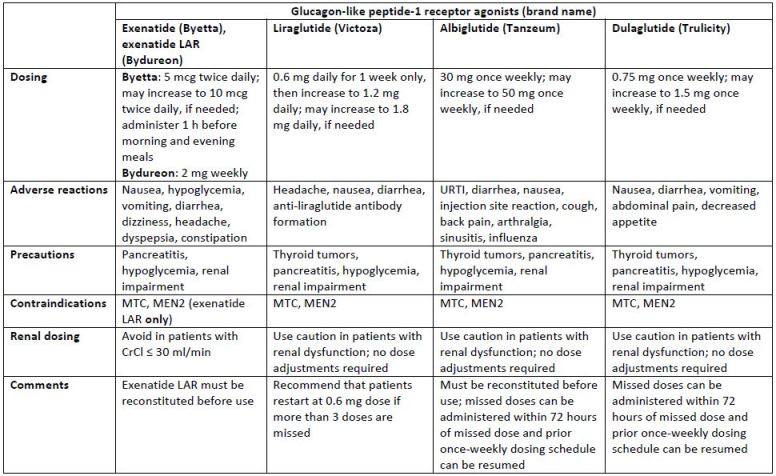
Combination Therapy With Insulins And Glp 1 Receptor Agonists

A Systematic Review And Meta Analysis Of The Efficacy Of Lixisenatide In The Treatment Of Patients With Type 2 Diabetes Schmidt 14 Diabetes Obesity And Metabolism Wiley Online Library

Table 4 From Efficacy And Safety Of Lixisenatide In Elderly 65 Years Old And Very Elderly 75 Years Old Patients With Type 2 Diabetes An Analysis From The Getgoal Phase Iii Programme Semantic Scholar

Initiating Injectable Treatment Of Type 2 Diabetes A Focus On Glucagon Like Peptide 1 Receptor Agonists Consultant360

Pdf Prandial Options To Advance Basal Insulin Glargine Therapy Testing Lixisenatide Plus Basal Insulin Versus Insulin Glulisine Either As Basal Plus Or Basal Bolus In Type 2 Diabetes The Getgoal Duo 2 Trial



